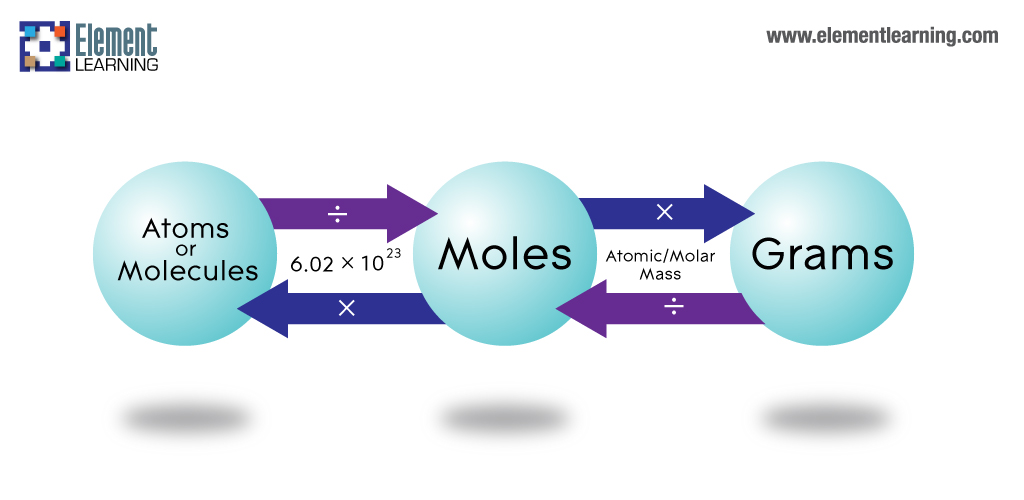
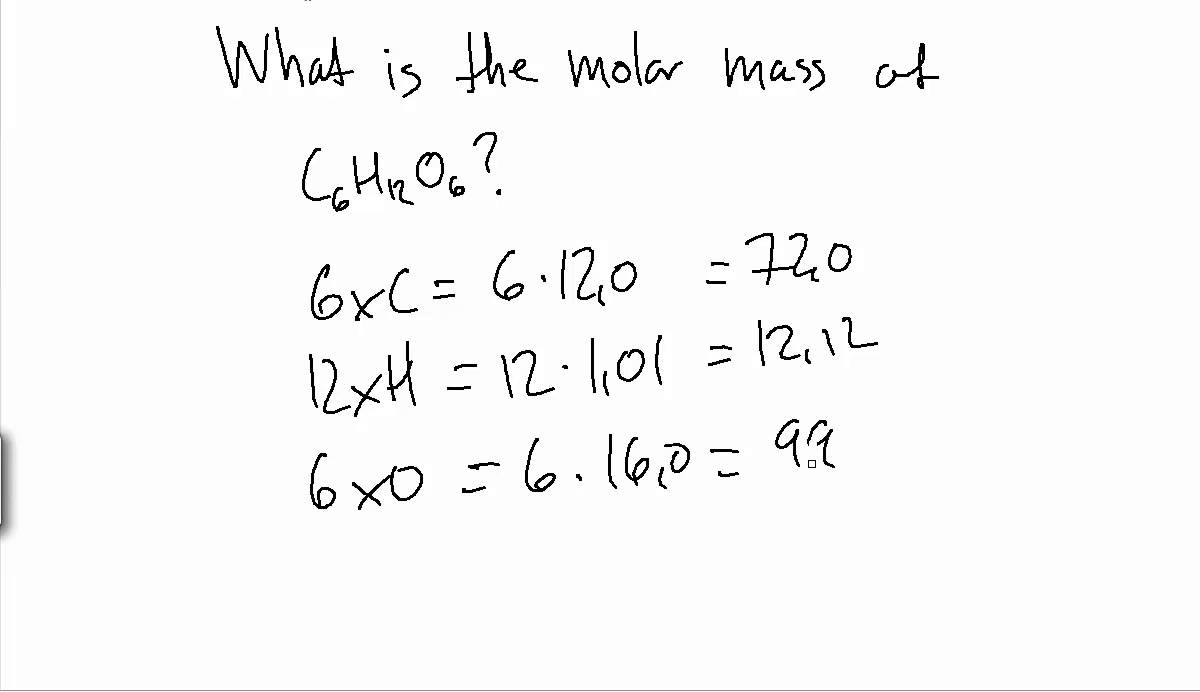The formula mass of a substance is the sum of the average atomic masses of each atom represented in the chemical formula and is expressed in atomic mass units. The formula mass of a covalent compound is also called the molecular mass. A convenient amount unit for expressing very large numbers of atoms or molecules is the mole. Experimental measurements have determined the number of entities composing 1 mole of substance to be 6.022 × 1023, a quantity called Avogadro's number. Due to the use of the same reference substance in defining the atomic mass unit and the mole, the formula mass and molar mass (g/mol) for any substance are numerically equivalent . The mole is a unit used to measure the number of atoms, molecules, or formula units in a given mass of a substance.
The mole is defined as the amount of substance that contains the number of carbon atoms in exactly 12 g of carbon-12 and consists of Avogadro's number (6.022 × 1023) of atoms of carbon-12. The molar mass of a substance is defined as the mass of 1 mol of that substance, expressed in grams per mole, and is equal to the mass of 6.022 × 1023 atoms, molecules, or formula units of that substance. That is, the molar mass of a substance is the mass of 6.022 × 1023 atoms, molecules, or formula units of that substance.
In each case, the number of grams in 1 mol is the same as the number of atomic mass units that describe the atomic mass, the molecular mass, or the formula mass, respectively. As you learned, the mass number is the sum of the numbers of protons and neutrons present in the nucleus of an atom. The mass number is an integer that is approximately equal to the numerical value of the atomic mass. Although the mass number is unitless, it is assigned units called atomic mass units .
The average mass of a monatomic ion is the same as the average mass of an atom of the element because the mass of electrons is so small that it is insignificant in most calculations. This is not much help in the laboratory for the typical chemist who only has a balance to weight out chemicals but needs to know how the number of atoms or molecules. Clearly something clever is needed but first let us briefly review how to calculated molecular masses. G/molIn chemistry, the molar mass of a chemical compound is defined as the mass of a sample of that compound divided by the amount of substance in that sample, measured in moles.
The molar mass is a bulk, not molecular, property of a substance. The molar mass is an average of many instances of the compound, which often vary in mass due to the presence of isotopes. Most commonly, the molar mass is computed from the standard atomic weights and is thus a terrestrial average and a function of the relative abundance of the isotopes of the constituent atoms on Earth. The molar mass is appropriate for converting between the mass of a substance and the amount of a substance for bulk quantities. To calculate the molecular mass of a compound, you need to know two things. The first is the molecular formula, and the second is the atomic mass number of each of the elements that comprise it.
The atomic mass number for each element is listed in atomic mass units under its symbol in the periodic table. This unit is defined in such a way that the mass number of each element corresponds to the mass of one mole of the element in grams. A mole equals Avogadro's number (6.02 x 1023) of atoms or molecules.
Of a substance is the sum of the average masses of the atoms in one molecule of a substance. It is calculated by adding together the atomic masses of the elements in the substance, each multiplied by its subscript in the molecular formula. Because the units of atomic mass are atomic mass units, the units of molecular mass are also atomic mass units.
The procedure for calculating molecular masses is illustrated in Example 1. The molar mass of any substance is numerically equivalent to its atomic or formula weight in amu. This relationship holds for all elements, since their atomic masses are measured relative to that of the amu-reference substance, 12C.
Extending this principle, the molar mass of a compound in grams is likewise numerically equivalent to its formula mass in amu (Figure 3.6). The mole is an important concept for talking about a very large number of things — 6.02 x 1023 of them to be exact. This module shows how the mole, known as Avogadro's number, is key to calculating quantities of atoms and molecules. It describes 19th-century developments that led to the concept of the mole, Topics include atomic weight, molecular weight, and molar mass. Sample equations illustrate how molar mass and Avogadro's number act as conversion factors to determine the amount of a substance and its mass. The mass of a mole of substance is called the molar mass of that substance.
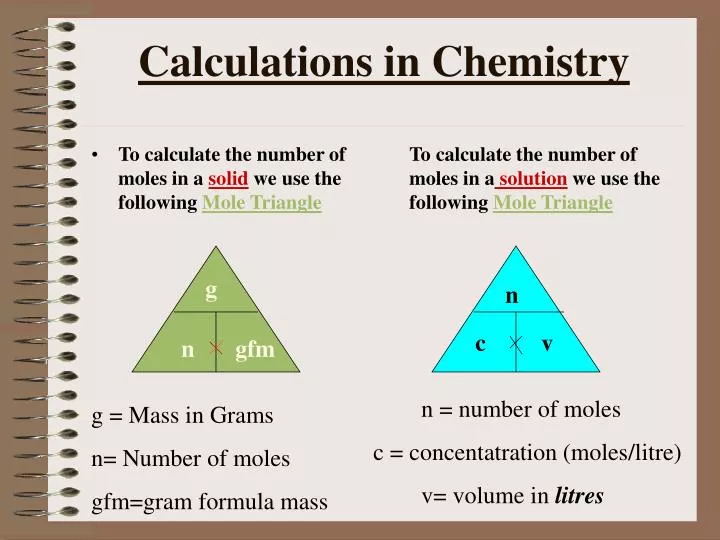
The molar mass is used to convert grams of a substance to moles and is used often in chemistry. The molar mass of an element is found on the periodic table, and it is the element's atomic weight in grams/mole (g/mol). If the mass of a substance is known, the number of moles in the substance can be calculated. Converting the mass, in grams, of a substance to moles requires a conversion factor of (one mole of substance/molar mass of substance). We can infer from the above equation that the quantity of a substance can be characterised by its mass or the number of molecules.
But, a chemical reaction equation indicates directly the number of atoms or molecules taking part in the reaction. Therefore, it is more convenient to refer to the quantity of a substance in terms of the number of its molecules or atoms, rather than their masses. One mole of any species is that quantity in number having a mass equal to its atomic or molecular mass in grams. In teh previous chapter, we discussed the concept of atomic mass. The molecular mass of a substance is the sum of the atomic masses of all the atoms in a molecule of the substance. It is therefore the relative mass of a molecule expressed in atomic mass units .
The mass of 1 mole of a substance is equal to its relative atomic or molecular mass in grams. The atomic mass of an element gives us the mass of one atom of that element in atomic mass units . To get the mass of 1 mole of atom of that element, that is, molar mass, we have to take the same numerical value but change the units from 'u' to 'g'. For polymers, proteins, DNA fragments, etc.While molar masses are almost always, in practice, calculated from atomic weights, they can also be measured in certain cases. Such measurements are much less precise than modern mass spectrometric measurements of atomic weights and molecular masses, and are of mostly historical interest. All of the procedures rely on colligative properties, and any dissociation of the compound must be taken into account.
In an earlier chapter, we described the development of the atomic mass unit, the concept of average atomic masses, and the use of chemical formulas to represent the elemental makeup of substances. These ideas can be extended to calculate the formula mass of a substance by summing the average atomic masses of all the atoms represented in the substance's formula. Each ion, or atom, has a particular mass; similarly, each mole of a given pure substance also has a definite mass. The mass of one mole of atoms of a pure element in grams is equivalent to the atomic mass of that element in atomic mass units or in grams per mole (g/mol).
Although mass can be expressed as both amu and g/mol, g/mol is the most useful system of units for laboratory chemistry. Many argue that modern chemical science began when scientists started exploring the quantitative as well as the qualitative aspects of chemistry. For example, Dalton's atomic theory was an attempt to explain the results of measurements that allowed him to calculate the relative masses of elements combined in various compounds. Understanding the relationship between the masses of atoms and the chemical formulas of compounds allows us to quantitatively describe the composition of substances. We use the mole to represent the amount of substances in chemistry because the numbers of atoms and molecules in each substance is so large.
The value given 6.022 x 1023is called Avagadro's number for the scientist that found the number of atoms in 12 grams of carbon 12. This means that the atomic mass or atomic weight of carbon is equal to exactly 1 mole of carbon. We can argue that modern chemical science began when scientists started exploring the quantitative as well as the qualitative aspects of chemistry.
In the case of 12C, we can see that the value for its molar mass and atomic mass both equal 12, although the units are different. While atomic mass is measured in amu, molar mass is measured in grams per mole. The identity of a substance is defined not only by the types of atoms or ions it contains, but by the quantity of each type of atom or ion. For example, water, H2O, and hydrogen peroxide, H2O2, are alike in that their respective molecules are composed of hydrogen and oxygen atoms. However, because a hydrogen peroxide molecule contains two oxygen atoms, as opposed to the water molecule, which has only one, the two substances exhibit very different properties.
This experimental approach required the introduction of a new unit for amount of substances, the mole, which remains indispensable in modern chemical science. The relationships between formula mass, the mole, and Avogadro's number can be applied to compute various quantities that describe the composition of substances and compounds. For example, if we know the mass and chemical composition of a substance, we can determine the number of moles and calculate number of atoms or molecules in the sample. Likewise, if we know the number of moles of a substance, we can derive the number of atoms or molecules and calculate the substance's mass.
Along with telling us the mass of one mole of an element, molar mass also acts as a conversion factor between the mass of a sample and the number moles in that sample. For example, 24 grams of 12C atoms would be equal to two moles since 24 grams divided by the mass of one mole equals 2. Further, Avogadro's number acts as the conversion factor for converting between the number of moles in a sample and the actual number of atoms or molecules in that sample. Extending our example, two moles of 12C atoms contains 2 times 6.02 x 1023 atoms, which equals 12.04 x 1023 atoms, which can be written as 1.204 x 1024 atoms. Note that the average masses of neutral sodium and chlorine atoms were used in this computation, rather than the masses for sodium cations and chlorine anions. This approach is perfectly acceptable when computing the formula mass of an ionic compound.
Even though a sodium cation has a slightly smaller mass than a sodium atom , this difference will be offset by the fact that a chloride anion is slightly more massive than a chloride atom . Moreover, the mass of an electron is negligibly small with respect to the mass of a typical atom. The few exceptions to this guideline are very light ions derived from elements with precisely known atomic masses. The molar mass of any substance is its atomic mass, molecular mass, or formula mass in grams per mole. Consistent with its definition as an amount unit, 1 mole of any element contains the same number of atoms as 1 mole of any other element. The masses of 1 mole of different elements, however, are different, since the masses of the individual atoms are drastically different.
The molar mass of an element is the mass in grams of 1 mole of that substance, a property expressed in units of grams per mole (g/mol) . A decade later, the same group added the mole into the metric system as a unit for the "amount of a substance." To define the exact amount that is in one mole unit, scientists again turned to 12C. To link the relative atomic mass scale to both absolute mass and moles, the group defined one mole as equal to the number of 12C atoms in 12 grams of 12C. The number of 12C atoms in 12 grams was experimentally determined to be 6.022 x 1023.
This value was named Avogadro's number , thereby replacing its earlier definition as the number of gas atoms in a cubic centimeter. However, in 1961, a new reference point was chosen by scientists with the International Committee for Weights and Measures, the group that defines the metric system's units of measurement. In place of 16O, the group decided to use the most common isotope of carbon, carbon-12 , as the reference. The group decided that the mass of one 12C atom would be set as 12 atomic mass units , and the atomic mass of the atoms of all other elements would be determined relative to 12C—the standard we still use today.
Atomic weight is useful for determining a substance's molecular weight—the average combined weight of a molecule's individual atoms. Like atomic weight, molecular weight is an averaged weight, based on the relative abundance of each atom's isotopes. However, as you can see on the periodic table, sulfur is listed as 32.07 amu—not 31.97!
This is because the periodic table lists atomic weights—the averages of the atomic mass for each one of the element's stable isotopes. Specifically, the periodic table lists the average atomic weight calculated based on the relative abundance of an element's different isotopes, as shown in the example with sulfur below. No matter how large or small the molecule, the procedure for calculating its molecular mass is the same. You look up the atomic mass of each of the elements in the formula, multiply it by the number of atoms of that element in the compound and add it to all the others. Atoms combine in various ways according to the number of electrons each has in its outer shell.
Ionic compounds, such as sodium chloride , may be composed of only one atom each of two different elements, and some covalent gases, such as hydrogen and oxygen are composed of two atoms of the same element. Some molecules, particularly those that form with carbon, can have a very large number of component atoms. The quantity of a substance that contains the same number of units (e.g., atoms or molecules) as the number of carbon atoms in exactly 12 g of isotopically pure carbon-12. Since the early 20th century, physicists had been developing a unified atomic mass scale—basically, a way for comparing the mass of an atom from one element to the mass of an atom from another element.
The atoms of all other elements were then compared to this 16O reference. While atomic mass and molar mass are numerically equivalent, keep in mind that they are vastly different in terms of scale, as represented by the vast difference in the magnitudes of their respective units . To appreciate the enormity of the mole, consider a small drop of water weighing about 0.03 g (see Figure 3.7).
Although this represents just a tiny fraction of 1 mole of water (~18 g), it contains more water molecules than can be clearly imagined. If the molecules were distributed equally among the roughly seven billion people on earth, each person would receive more than 100 billion molecules. The molecular weight of a compound is the sum of the atomic weights of the atoms in the molecules that form these compounds.
The MOLE is a unit of measurement that is the amount of a pure substance containing the same number of chemical units (atoms, molecules etc.) as there are atoms in exactly 12 grams of carbon-12 (i.e., 6.022 X 1023). The relative formula mass of a compound is calculated by adding together the relative atomic mass values for all the atoms in its formula. The mole provides a bridge between the atomic world and the laboratory .
It allows determination of the number of molecules or atoms by weighing them. A mole of C-12 by definition weighs exactly 12 g and Avogadro's number is determined by counting the number of atoms. Avogadro's number is the fundamental constant that is least accurately determined. The mole is an amount unit similar to familiar units like pair, dozen, gross, etc.
It provides a specific measure of the number of atoms or molecules in a bulk sample of matter. A mole is defined as the amount of substance containing the same number of discrete entities as the number of atoms in a sample of pure 12C weighing exactly 12 g. One Latin connotation for the word "mole" is "large mass" or "bulk," which is consistent with its use as the name for this unit.
The mole provides a link between an easily measured macroscopic property, bulk mass, and an extremely important fundamental property, number of atoms, molecules, and so forth. The characteristic molar mass of an element is simply the atomic mass in g/mol. However, molar mass can also be calculated by multiplying the atomic mass in amu by the molar mass constant (1 g/mol). To calculate the molar mass of a compound with multiple atoms, sum all the atomic mass of the constituent atoms.